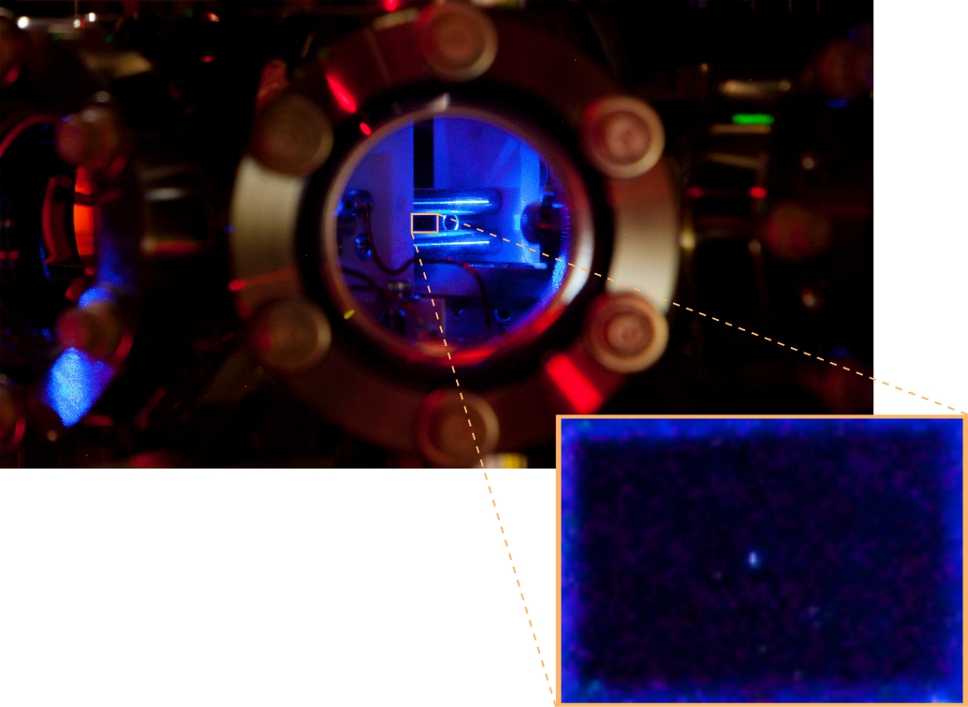
Mingyu Fan took a photograph of a single radium ion scattering light as it hovered in free space where it was trapped by electric fields. In the primary picture you can see our vacuum chamber and trapping apparatus. When you look through the window of our vacuum chamber and zoom in to the highlighted yellow region you find floating in empty space (the black rectangle) a radium atom. The camera captures the light scattered by a radium ion over several pixels, resulting in the little blue dot in the center of the image.
Our research both studies and utilizes radium ions and it requires exacting levels of control. We need our ions to stay fixed in space for 10’s of hours so that we can both manipulate and detect the radium ion with laser light. For example, while holding the ion with electric fields we apply two lasers at different wavelengths, 468 nm (the blue light in the picture) and 1079 nm (infrared light that is invisible to your eye), to laser-cool the ion. This level of control requires a lot of careful work and creativity, primarily by Mingyu and Craig.
A challenge of portrait photography is getting your subject to stay still under the proper conditions, etc. With the time that has been put into our scientific apparatus it is easy (now!) to have our subject sit still, typically for an entire night of automated measurements, and the only remaining challenge is keeping the camera steady for a long exposure to collect the faint bits of light emitted from a single radium atom.
Unlike most atoms you might be familiar with radium is radioactive. This means you are looking at a picture of a young atom (it has not decayed yet). This particular isotope, radium 226, has a half-life of 1600 years, so it is almost certainly older than you are, but probably younger than The Great Pyramid and definitely a little baby compared to the dinosaurs.